SeqShop: Variant Calling and Filtering for SNPs Practical, June 2014
Introduction
See the introductory slides for an intro to this tutorial.
Goals of This Session
- What we want to learn
- How to generate filtered variant calls for SNPs from BAMs
- Basic variant call file format (VCF)
- How to examine the variants at particular genomic positions
- How to evaluate the quality of SNP calls
Login to the seqshop-server Linux Machine
This section will appear redundantly in each session. If you are already logged in or know how to log in to the server, please skip this section
- Login to the windows machine
- The username/password for the Windows machine should be written on the right-hand monitor
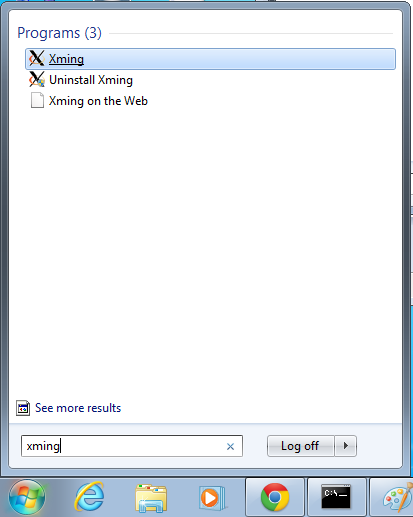
- Start xming so you can open external windows on our Linux machine
- Start->Enter "Xming" in the search and select "Xming" from the program list
- Nothing will happen, but Xming was started.
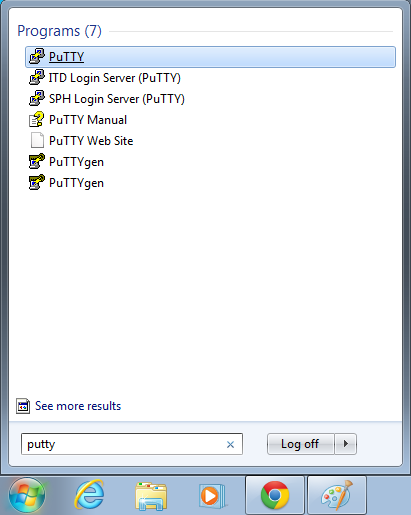
- Open putty
- Start->Enter "putty" in the search and select "PuTTY" from the program list
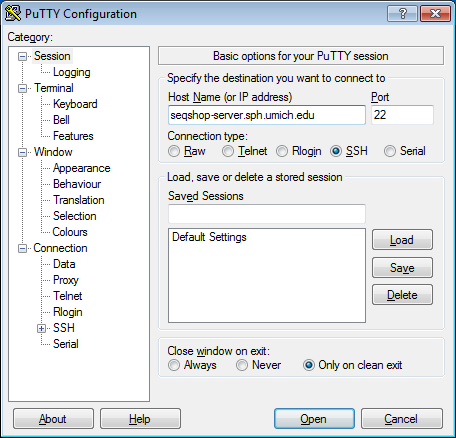
- Configure PuTTY in the PuTTY Configuration window
- Host Name:
seqshop-server.sph.umich.edu - Setup to allow you to open external windows:
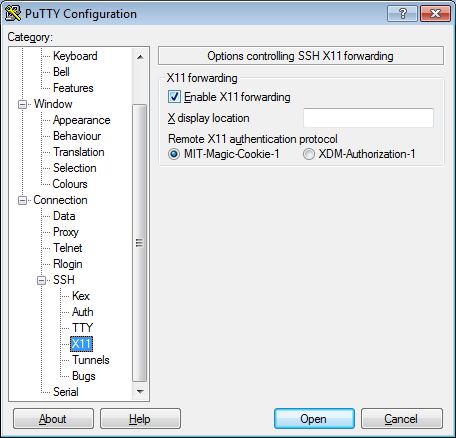
- In the left pannel: Connection->SSH->X11
- Add a check mark in the box next to
Enable X11 forwarding - Click
Open - If it prompts about a key, click
OK - Enter your provided username & password as provided
You should now be logged into a terminal on the seqshop-server and be able to access the test files.
- If you need another terminal, repeat from step 3.
Login to the seqshop Machine
So you can each run multiple jobs at once, we will have you run on 4 different machines within our seqshop setup.
- You can only access these machines after logging onto seqshop-server
3 users logon to:
ssh -X seqshop1
3 users logon to:
ssh -X seqshop2
2 users logon to:
ssh -X seqshop3
2 users logon to:
ssh -X seqshop4
Setup your run environment
This is the same setup you did for the previous tutorial, but you need to redo it each time you log in. It will setup some environment variables to point you to:
- GotCloud program
- Tutorial input files
- Setup an output directory
- It will leave your output directory from the previous tutorial in tact.
source /home/mktrost/seqshop/setup.txt
- You won't see any output after running
source- It silently sets up your environment
- If you want to view the detail of the set up, type
less /home/mktrost/seqshop/setup.txt
and press 'q' to finish.
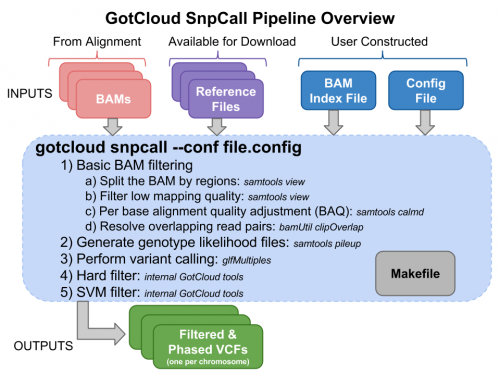
Examining GotCloud SnpCall Input files
Sequnce Alignment Files: BAM Files
Per sample BAM files contain sequence reads that are mapped to positions in the genome.
For a reminder on how to look at/read BAM files, see: SeqShop Aligment: BAM Files
For this tutorial, we will use the 4 BAMs produced in the SeqShop: Sequence Mapping and Assembly Practical as well as with 58 BAMs that were pre-aligned to that 1MB region of chromosome 22.
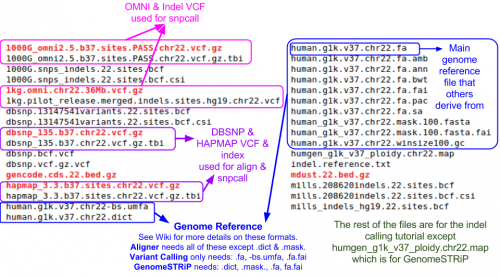
Reference Files
Reference files can be downloaded with GotCloud or from other sources.
- For this practical, I already downloaded them for you.
- See GotCloud: Genetic Reference and Resource Files for more information on downloading/generating reference files
For GotCloud snpcall, you need:
- Reference genome FASTA file
- Contains the reference base for each position of each chromosome
- Used to compare bases in sequence reads to the reference positions they mapped to
- Used to identify SNPs/variations in the sequence reads
- Additional information on the FASTA format: http://en.wikipedia.org/wiki/FASTA_format
- Contains the reference base for each position of each chromosome
- VCF (variant call format) files with chromosomes/positions
- indel - contains known insertions & deletions to help with filtering
- omni - used as likely true positives for SVM filtering
- hapmap - used as likely true positives for SVM filtering and for generating summary statistics
- dbsnp - used for generating summary statistics
We looked at them yesterday, but you can take another look at the chromosome 22 reference files included for this tutorial:
ls ${REF}
GotCloud BAM Index File
The BAM index file points GotCloud to the BAM files
- generated by the alignment pipeline
Look at the BAM index file the alignment pipeline generated
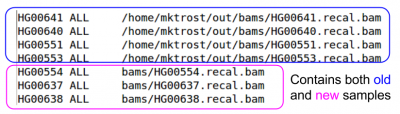
cat ${OUT}/bam.index
- What is the path to the BAM file for sample HG00640?
The alignment pipeline only processed 4 samples, but for snpcall, we want to run on 62 samples.
- The other 58 samples were already aligned:
ls ${IN}/bams
Look at the BAM index for those BAMs:
less ${IN}/bams/bam.index
Remember, use 'q' to exit out of less
q
- Do you notice a difference between this index and yours?
We need to add these BAMs to our index
- Append the bam.index from the pre-aligned BAMs to the one you generated from the alignment pipeline
cat ${IN}/bams/bam.index >> ${OUT}/bam.index
- ">>" will append to the file that follows it
Verify your BAM index contains the additional BAMs
less ${OUT}/bam.index
Remember, use 'q' to exit out of less
q
- Do you see both sets of BAMs?
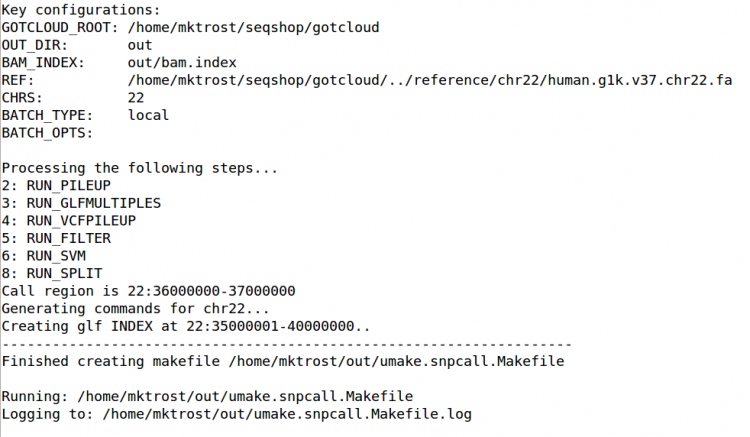
GotCloud Configuration File
We will use the same configuration file as we used yesterday in GotCloud Align.
See SeqShop: Alignment: GotCloud Configuration File for more details
- Note we want to limit snpcall to just chr22 so the configuration already has
CHRS = 22(default was 1-22 & X).
For more information on configuration, see: GotCloud snpcall: Configuration File
- Contains information on how to configure for exome/targeted sequencing
Run GotCloud SnpCall
Now that we have all of our input files, we need just a simple command to run:
${GC}/gotcloud snpcall --conf ${IN}/gotcloud.conf --numjobs 4 --region 22:36000000-37000000
- --numjobs tells GotCloud how many jobs to run in parallel
- Depends on your system
- --region 22:36000000-37000000
- The sample files are just a small region of chromosome 22, so to save time, we tell GotCloud to ignore the other regions
This should take about 5 minutes to run.
- After about 4 minutes of running, GotCloud snpcall will output some text to the screen. Don't worry, that is expected and is just output from some of the intermediate tools.
- It should end with a line like:
Commands finished in 289 secs with no errors reported
If you cancelled GotCloud part way through, just rerun your GotCloud command and it will pick up where it left off.
Examining GotCloud SnpCall Output
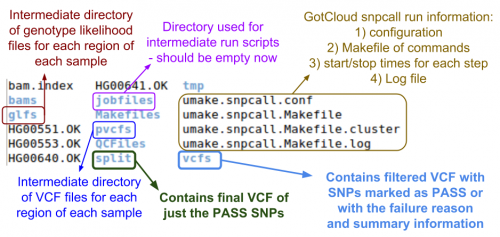
Let's look at the output directory:
ls ${OUT}
- Do you see any new files or directories?
Let's look at the vcfs directory:
ls ${OUT}/vcfs
Just a chr22 directory, so look inside of there:
ls ${OUT}/vcfs/chr22
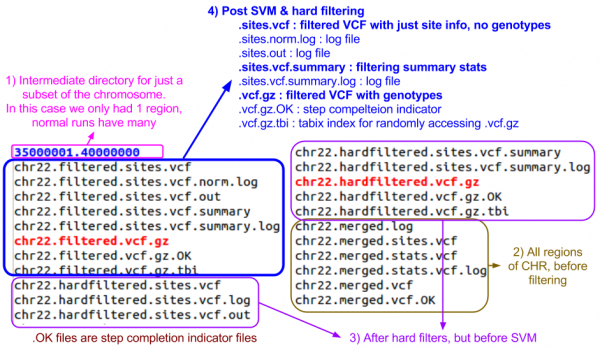
- Can you identify the final filtered VCF and the associated summary file?
- Answer & annotated directory listing:
Now, let's look in the split directory for the VCF with just the passing variants:
ls ${OUT}/split/chr22
- Which file do you think is the one you want?
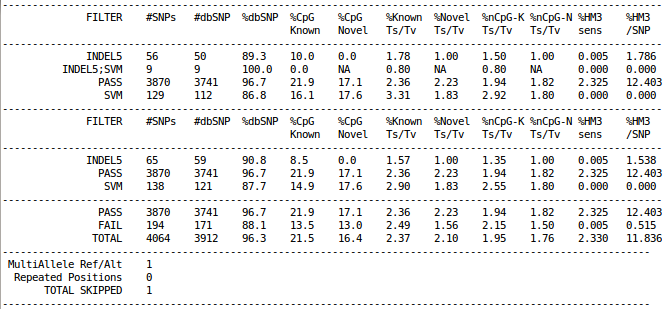
Filtering Summary Statistics
cat ${OUT}/vcfs/chr22/chr22.filtered.sites.vcf.summary
Filtered VCF
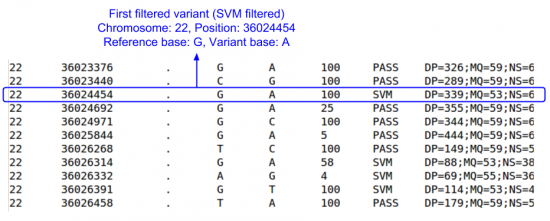
Let's look at the filtered sites file.
less -S ${OUT}/vcfs/chr22/chr22.filtered.sites.vcf
- Scroll down until you find some variants.
- Use space bar to jump a full page
- Use down arrow to move down one line
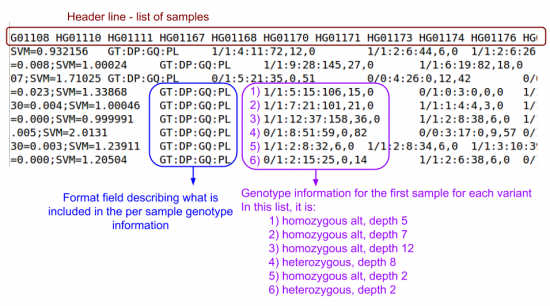
- Scroll right: lots of info fields, but no per sample genotype information
- What is the first filtered out variant that you find & what filter did it fail?
Remember, use 'q' to exit out of less
q
Now, let's look at the filtered file with genotypes.
zless -S ${OUT}/vcfs/chr22/chr22.filtered.vcf.gz
- Scroll down until you find some variants.
- Use space bar to jump a full page
- Use down arrow to move down one line
- Scroll right until you should see per sample genotype information
Passing SNPs
Let's look at the file of just the pass sites:
zless -S ${OUT}/split/chr22/chr22.filtered.PASS.vcf.gz
- Scroll down: they all look like they
PASS
Let's check if they are all PASS.
zcat ${OUT}/split/chr22/chr22.filtered.PASS.vcf.gz |grep -v "^#"| cut -f 7| grep -v "PASS"
It will return nothing since there are no non-passing variants in this file.
- Want an explanation of this command?
- zcat ...: uncompress the zipped VCF
- '|' : this takes the output of one command and sends it as input to the next
- grep -v "^#" : exclude any lines that start with "#" - headers
- cut -f 7 : extract the FILTER column (the 7th column)
- grep -v "PASS" : exclude any rows that have a "PASS" in the FILTER column
Compare that to the filtered file we looked at before:
zcat ${OUT}/vcfs/chr22/chr22.filtered.vcf.gz |grep -v "^#"| cut -f 7| grep -v "PASS"
- Do you see any filters?
- Answer
- Yes
- It should have scrolled and you should see filters like:
- INDEL5;SVM
- INDEL5
- SVM
- It should have scrolled and you should see filters like:
GotCloud Genotype Refinement
To improve the quality of the genotypes, we run a genotype refinement pipeline.
This pipeline runs Beagle & thunder.
Genotype Refinement Input
The GotCloud genotype refinement pipeline takes as input ${OUT}/split/chr22/chr22.filtered.PASS.vcf.gz (the VCF file of PASS'ing SNPs from snpcall.
The bam index and the configuration file we used for GotCloud snpcall will tell GotCloud genotype refinement everything it needs to know, so no new input files need to be prepared.
Note: the configuration file overrides the THUNDER command to make it go faster than the default settings so the tutorial will run faster:

Running GotCloud Genotype Refinement
Since everything is setup, just run the following command (very similar to snpcall).
${GC}/gotcloud ldrefine --conf ${IN}/gotcloud.conf --numjobs 2 --region 22:36000000-37000000
- Beagle will take about 2-3 minutes to complete
- Thunder will automatically run and will take another 3-4 minutes
Genotype Refinement Output
- What's new in the output directory?
- Answer
beagledirectory : Beagle outputthunderdirectory : Thunder outputumake.beagle.conf: Configuration values used for GotCloud beagleumake.beagle.Makefile: GNU makefile for commands run as part of GotCloud beagleumake.beagle.Makefile.log: Log of the GotCloud beagle runumake.thunder.*files : Contain the configuration & steps used in GotCloud thunder
Let's take a look at that interesting location we found in the alignment tutorial : chromosome 22, positions 36907000-36907100
Use tabix to extract that from the VCFs:
${GC}/bin/tabix ${OUT}/thunder/chr22/ALL/thunder/chr22.filtered.PASS.beagled.ALL.thunder.vcf.gz 22:36907000-36907100 |less -S
Remember, type 'q' to quit less.
q
- Are there any variants in this region?
- Answer:
- Yes!
- Positions:
36907001; Ref: T, Alt: C - that's what we saw before36907098; Ref: T, Alt: C - that's what we saw before
- What is HG00551's genotype at these positions?
- First check which sample number HG00551 is:
zcat ${OUT}/thunder/chr22/ALL/thunder/chr22.filtered.PASS.beagled.ALL.thunder.vcf.gz |grep "#CHROM"
- That will help you figure out it's genotype.
- Rerun the tabix command and scroll to find HG00551's genotype:
${GC}/bin/tabix ${OUT}/thunder/chr22/ALL/thunder/chr22.filtered.PASS.beagled.ALL.thunder.vcf.gz 22:36907000-36907100 |less -S
- Answer:
- It is the first sample
0|1: Heterozygous1|1; Homozygous Alt (C)
Remember, type 'q' to quit less.
q