Difference between revisions of "Tutorial: GotCloud"
| Line 7: | Line 7: | ||
The variant calling pipeline processes the BAMs file produced by the alignment pipeline, generating an initial list of polymorphic sites and genotypes stored in a [http://www.1000genomes.org/wiki/Analysis/Variant%20Call%20Format/vcf-variant-call-format-version-41 VCF (Variant Call Format) file] and then uses haplotype information to refine these genotypes in an updated VCF file. | The variant calling pipeline processes the BAMs file produced by the alignment pipeline, generating an initial list of polymorphic sites and genotypes stored in a [http://www.1000genomes.org/wiki/Analysis/Variant%20Call%20Format/vcf-variant-call-format-version-41 VCF (Variant Call Format) file] and then uses haplotype information to refine these genotypes in an updated VCF file. | ||
| + | |||
| + | After variant calling, there is an optional step to further filter the variants using a [[SVM Filtering|Support Vector Machine (SVM)]]. This feature is in development and will soon be added to gotcloud and this tutorial. | ||
This tutorial then demonstrates how [[EPACTS|EPACTS (Efficient and Parallelizable Association Container Toolbox)]] can be used to perform statistical tests to identify genome-wide association from sequence data. | This tutorial then demonstrates how [[EPACTS|EPACTS (Efficient and Parallelizable Association Container Toolbox)]] can be used to perform statistical tests to identify genome-wide association from sequence data. | ||
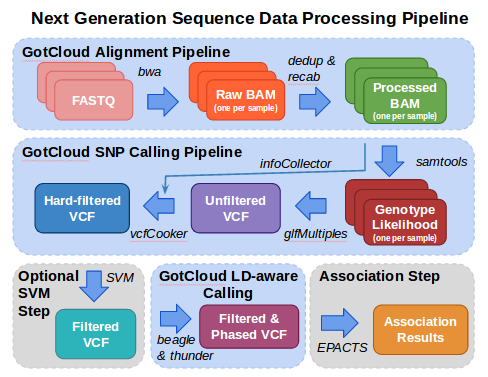
[[File:GotCloudDiagram.png]] | [[File:GotCloudDiagram.png]] | ||
| + | |||
| + | [[GotCloud]] incorporates the alignment and variant calling pipelines into one easy to use tool. GotCloud can run on a user's computer, on an instance in a compute cloud, and can even split the work up onto a cluster of machines or instances. This tutorial is just a small test that just runs on the machine the commands are run on. | ||
== STEP 1 : Setup GotCloud == | == STEP 1 : Setup GotCloud == | ||
| + | |||
| + | [[GotCloud]] has been developed and tested on Linux Ubuntu 12.10 and 12.04.2 LTS but has not been tested on other Linux operating systems. It is not available for Windows. If you do not have your own set of machines to run on, GotCloud is also available for Ubuntu running on the Amazon Elastic Compute Cloud, see [[Amazon_Snapshot]] for more information. | ||
| + | |||
=== Step 1a: Setup Environment === | === Step 1a: Setup Environment === | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | We will use 3 different directories for this tutorial and will use the following variables to stand for these directories: | |
| + | # $GCHOME : path to the directory where gotcloud is installed | ||
| + | # $GCDATA : path to the directory where the example data is installed | ||
| + | # $GCOUT : path to your output directory | ||
| + | |||
| + | The following steps will set these environment variables in your Linux terminal allowing you to type $GCHOME to specify the path to gotcloud instead of having to type the entire path (absolute path). | ||
| + | |||
| + | Note: These settings will be local to that specific terminal. If you open a new terminal you will need to set the variables again in that terminal. | ||
| + | |||
| + | There are two different ways to set these variables and they depend on what type of shell your terminal is using. The shell you are using does not matter, other than for using the appropriate commands for setting the variables. To determine which shell your terminal is running, the following command will display your shell type, so type the following in your terminal: | ||
| + | |||
ps -p $$ -ocomm= | ps -p $$ -ocomm= | ||
| − | + | If your shell is bash or sh, set your variables using: | |
export GCHOME=~/gotcloud | export GCHOME=~/gotcloud | ||
export GCDATA=~/gotcloudExample | export GCDATA=~/gotcloudExample | ||
export GCOUT=~/gotcloudTutorial | export GCOUT=~/gotcloudTutorial | ||
| − | + | If your shell is csh, tcsh, set your variables using: | |
setenv GCHOME ~/gotcloud | setenv GCHOME ~/gotcloud | ||
setenv GCDATA ~/gotcloudExample | setenv GCDATA ~/gotcloudExample | ||
setenv GCOUT ~/gotcloudTutorial | setenv GCOUT ~/gotcloudTutorial | ||
| + | |||
| + | If the directories specified above do not reflect where gotcloud and/or the example data is installed or where you want your output to go, then replace those directories with the full paths to the appropriate directories. | ||
| + | |||
| + | After setting these variables, you can copy and paste the rest of the commands in the tutorial. If you do not want to use variables, you can type the commands in with the appropriate paths specified. | ||
| + | |||
=== Step 1b: Install GotCloud === | === Step 1b: Install GotCloud === | ||
| − | In order to run this tutorial, you need to make sure you have GotCloud | + | In order to run this tutorial, you need to make sure you have GotCloud installed on your system. |
If you have root and would like to install gotcloud on your system, follow: [[GotCloud#Install_GotCloud_Software| root access installation instructions]] | If you have root and would like to install gotcloud on your system, follow: [[GotCloud#Install_GotCloud_Software| root access installation instructions]] | ||
Otherwise, you can install it in your own directory: | Otherwise, you can install it in your own directory: | ||
| − | # Create & | + | # Create & change to the directory where you want gotcloud installed |
# Download the gotcloud tar from the ftp site. | # Download the gotcloud tar from the ftp site. | ||
# Extract the tar | # Extract the tar | ||
| − | # Build the source | + | # Build (compile) the source |
| + | #* Note: as the source builds, many messages will scroll through your terminal. You may even see some warnings. These messages are normal and expected. As long as the build does not end with an error, you have successfully built the source. | ||
mkdir -p $GCHOME; cd $GCHOME | mkdir -p $GCHOME; cd $GCHOME | ||
| Line 49: | Line 68: | ||
cd $GCHOME/src; make # Build source | cd $GCHOME/src; make # Build source | ||
| + | GotCloud requires the following tools to be installed. | ||
| + | You can run $GCHOME/scripts/check_requirements.sh | ||
| + | ...TBD – put in required programs/tools. | ||
| + | * java (java-common default-jre on ubuntu) | ||
| + | * make (make on ubuntu) | ||
| + | * libssl (libssl0.9.8 on ubuntu) | ||
=== Step 1c: Install Example Dataset === | === Step 1c: Install Example Dataset === | ||
| − | Our dataset consists of 60 individuals from GBR sequenced by the 1000 Genomes Project. These individuals have been sequenced to an average depth of about 4x. | + | Our dataset consists of 60 individuals from Great Britain (GBR) sequenced by the 1000 Genomes Project. These individuals have been sequenced to an average depth of about 4x. |
| + | |||
| + | To conserve time and disk-space, our analysis will focus on a small region on chromosome 20, 42900000 - 43200000. | ||
| − | + | The tutorial will run the alignment pipeline on 2 of the individuals (HG00096, HG00100). The fastqs used for this step are reduced to reads that fall into our target region. | |
| − | The | + | The tutorial will then used previously aligned/mapped reads for the full 60 individuals to generate a list of polymorphic sites and estimate accurate genotypes at each of these sites. |
| − | The example dataset we'll be using is available at: ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar | + | The example dataset we'll be using is available at: ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar |
| − | # Create & Change directory to where you want to install the Tutorail data | + | # Create & Change directory to where you want to install the Tutorail data |
| − | # Download the dataset tar from the ftp site | + | # Download the dataset tar from the ftp site |
| − | # Extract the tar | + | # Extract the tar |
| − | mkdir -p $GCDATA; cd $GCDATA | + | mkdir -p $GCDATA; cd $GCDATA |
| − | wget ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar # Download | + | wget ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar # Download |
| − | tar xvf gotcloudExample.tar --strip 1 # Extract | + | tar xvf gotcloudExample.tar --strip 1 # Extract |
| − | == STEP 2 : Run GotCloud Alignment Pipeline == | + | == STEP 2 : Run GotCloud Alignment Pipeline == |
The first step in processing next generation sequence data is mapping the reads to the reference genome, generating per sample BAM files. | The first step in processing next generation sequence data is mapping the reads to the reference genome, generating per sample BAM files. | ||
| − | The alignment pipeline has multiple built-in steps to generate BAMs: | + | The alignment pipeline has multiple built-in steps to generate BAMs: |
| − | # Align the fastqs to the reference genome | + | # Align the fastqs to the reference genome |
| − | #* handles both single & paired end | + | #* handles both single & paired end |
| − | # Merge the results from multiple fastqs into 1 file per sample | + | # Merge the results from multiple fastqs into 1 file per sample |
| − | # Mark Duplicate Reads are marked | + | # Mark Duplicate Reads are marked |
| − | # Recalibrate Base Qualities | + | # Recalibrate Base Qualities |
| − | This processing results in 1 BAM file per sample. | + | This processing results in 1 BAM file per sample. |
| − | The alignment pipeline also includes Quality Control (QC) steps: | + | The alignment pipeline also includes Quality Control (QC) steps: |
| − | # Visualization of various quality measures (QPLOT) | + | # Visualization of various quality measures (QPLOT) |
| − | # Screen for sample contamination & swap (VerifyBamID) | + | # Screen for sample contamination & swap (VerifyBamID) |
| − | Run the alignment pipeline (the example aligns 2 samples) : | + | Run the alignment pipeline (the example aligns 2 samples) : |
| − | $GCHOME/gotcloud align --conf $GCDATA/[[Alignment Configuration File|GBR60align.conf]] --outdir $GCOUT | + | $GCHOME/gotcloud align --conf $GCDATA/[[Alignment Configuration File|GBR60align.conf]] --outdir $GCOUT |
Upon successful completion of the alignment pipeline (about 1-2 minutes), you will see the following message: | Upon successful completion of the alignment pipeline (about 1-2 minutes), you will see the following message: | ||
| − | Processing finished in nn secs with no errors reported | + | Processing finished in nn secs with no errors reported |
| + | |||
| + | The final BAM files produced by the alignment pipeline are: | ||
| + | ls $GCOUT/bams | ||
| + | In this directory you will see: | ||
| + | * BAM (.bam) files - 1 per sample | ||
| + | ** HG00096.recal.bam | ||
| + | ** HG00100.recal.bam | ||
| + | * BAM index files (.bai) – 1 per sample | ||
| + | ** HG00096.recal.bam.bai | ||
| + | ** HG00100.recal.bam.bai | ||
| + | * BAM checksum files (.md5) – 1 per sample | ||
| + | ** HG00096.recal.bam.md5 | ||
| + | ** HG00100.recal.bam.md5 | ||
| + | * Indicator flies that the step completed successfully: | ||
| + | ** HG00096.recal.bam.done | ||
| + | ** HG00100.recal.bam.done | ||
| + | |||
| + | The Quality Control (QC) files are: | ||
| + | ls $GCOUT/QCFiles | ||
| + | In this directory you will see: | ||
| + | * VerifyBamID output files: | ||
| + | ** HG00096.genoCheck.depthRG | ||
| + | ** HG00096.genoCheck.depthSM | ||
| + | ** HG00096.genoCheck.selfRG | ||
| + | ** HG00096.genoCheck.selfSM | ||
| + | ** HG00100.genoCheck.depthRG | ||
| + | ** HG00100.genoCheck.depthSM | ||
| + | ** HG00100.genoCheck.selfRG | ||
| + | ** HG00100.genoCheck.selfSM | ||
| + | |||
| + | * VerifyBamID step completion files – 1 per sample | ||
| + | ** HG00096.genoCheck.done | ||
| + | ** HG00100.genoCheck.done | ||
| − | + | * QPLOT output files | |
| − | + | ** HG00096.qplot.R | |
| + | ** HG00096.qplot.stats | ||
| + | ** HG00100.qplot.R | ||
| + | ** HG00100.qplot.stats | ||
| − | + | * QPLOT step completion files – 1 per sample | |
| + | ** HG00096.qplot.done | ||
| + | ** HG00100.qplot.done | ||
| − | + | For information on the VerifyBamID output, see: [[Understanding VerifyBamID output]] | |
| − | |||
| − | |||
| − | + | For information on the QPLOT output, see: [[Understanding QPLOT output]] | |
| − | |||
| − | + | == STEP 3 : Run GotCloud Variant Calling Pipeline == | |
| + | The next step is to analyze BAM files by calling SNPs and generating a VCF file containing the variant calls. | ||
| − | + | The variant calling pipeline has multiple built-in steps to generate BAMs: | |
| − | + | # Filter out reads with low mapping quality | |
| + | # Per Base Alignment Quality Adjustment (BAQ) | ||
| + | # Resolve overlapping paired end reads | ||
| + | # Generate genotype likelihood files | ||
| + | # Perform variant calling | ||
| + | # Extract features from variant sites | ||
| + | # Perform variant filtering | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | To speed variant calling, each chromosome is broken up into smaller regions which are processed separately. While initially split by sample, the per sample data gets merged and is processed together for each region. These regions are later merged to result in a single Variant Call File (VCF) per chromosome. | |
| − | Run the variant calling pipeline: | + | Run the variant calling pipeline: |
| − | $GCHOME/gotcloud snpcall --conf [[GBR60vc.conf]] --outdir $GCOUT --numjobs 2 --region 20:42900000-43200000 | + | $GCHOME/gotcloud snpcall --conf [[GBR60vc.conf]] --outdir $GCOUT --numjobs 2 --region 20:42900000-43200000 |
Upon successful completion of the variant calling pipeline (about 3-4 minutes), you will see the following message: | Upon successful completion of the variant calling pipeline (about 3-4 minutes), you will see the following message: | ||
| − | Commands finished in nnn secs with no errors reported | + | Commands finished in nnn secs with no errors reported |
| − | + | On SNP Call success, the VCF files of interest are: | |
| − | ls $GCOUT/ | + | ls $GCOUT/vcfs/chr20/chr20.filtered* |
| − | + | This gives you the following files: | |
| − | + | * '''chr20.filtered.vcf.gz ''' - vcf for whole chromosome after it has been run through filters and marked with PASS/FAIL including per sample information | |
| + | * chr20.filtered.sites.vcf - vcf for whole chromosome after it has been run through filters and marked with PASS/FAIL without the per sample information | ||
| + | * chr20.filtered.sites.vcf.log - log file | ||
| + | * chr20.filtered.sites.vcf.summary - summary of filters applied | ||
| + | * chr20.filtered.vcf.gz.OK - indicator that the filtering completed successfully | ||
| + | * chr20.filtered.vcf.gz.tbi - index file for the vcf file | ||
| − | + | Also in the $GCOUT/vcfs/chr20 directory are intermediate files: | |
| − | + | * the whole chromosome variant calls prior to any filtering: | |
| + | ** chr20.merged.sites.vcf - no sample information | ||
| + | ** chr20.merged.stats.vcf | ||
| + | ** chr20.merged.vcf - includes sample information | ||
| + | ** chr20.merged.vcf.OK - indicator that the step completed successfully | ||
| + | * 40000001.45000000 subdirectory contains the data for just that region. | ||
| − | Run the LD-aware genotype refinement pipeline: | + | The $GCOUT/split/chr20 folder contains a VCF with just the sites that pass the filters. |
| − | $GCHOME/gotcloud ldrefine --conf [[GBR60vc.conf]] --outdir $GCOUT --numjobs 2 | + | Ls $GCOUT/split/chr20/ |
| + | * '''chr20.filtered.PASS.vcf.gz ''' – vcf of just sites that pass all filters | ||
| + | * chr20.filtered.PASS.split.1.vcf.gz - intermediate file | ||
| + | * chr20.filtered.PASS.split.err - log file | ||
| + | * chr20.filtered.PASS.split.vcflist - list of intermediate files | ||
| + | * subset.OK | ||
| + | |||
| + | In addition to the vcfs subdirectory, there are additional intermediate files/directories: | ||
| + | * glfs – holds genotype likelihood format [[GLF]] files split by chromosome, region, and sample | ||
| + | * pvcfs – holds intermediate vcf files split by chromosome and region | ||
| + | |||
| + | Note: the tutorial does not produce a target directory, but if you run with targeted data, you may see that. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == STEP 4 : Run GotCloud Genotype Refinement Pipeline == | ||
| + | The next step is to perform genotype refinement using linkage disequilibrium information using [http://faculty.washington.edu/browning/beagle/beagle.html Beagle] & [[ThunderVCF]]. | ||
| + | |||
| + | Run the LD-aware genotype refinement pipeline: | ||
| + | $GCHOME/gotcloud ldrefine --conf [[GBR60vc.conf]] --outdir $GCOUT --numjobs 2 | ||
Upon successful completion of this pipeline, you will see the following message: | Upon successful completion of this pipeline, you will see the following message: | ||
| − | Commands finished in nnn secs with no errors reported | + | Commands finished in nnn secs with no errors reported |
| + | |||
| + | The output from the beagle step of the genotype refinement pipeline is found in: | ||
| + | ls $GCOUT/beagle/chr20/chr20.filtered.PASS.beagled.vcf.gz $GCOUT/beagle/chr20/chr20.filtered.PASS.beagled.vcf.gz.tbi | ||
| + | |||
| + | The output from the thunderVcf (final step) of the genotype refinement pipeline is found in: | ||
| + | ls $GCOUT/thunder/chr20/GBR/chr20.filtered.PASS.beagled.GBR.thunder.vcf.gz $GCOUT/thunder/chr20/GBR/chr20.filtered.PASS.beagled.GBR.thunder.vcf.gz.tbi | ||
| − | + | == STEP 5 : Run Support Vector Machine (SVM) Pipeline == | |
| − | |||
| − | |||
| − | |||
| − | == STEP | + | == STEP 6 : Run GotCloud Association Analysis Pipeline == |
| − | /net/fantasia/home/hmkang/bin/epacts/bin/epacts single --vcf $GCOUT/vcfs/chr20/chr20.filtered.vcf.gz --ped $GCDATA/test.GBR60.ped --out EPACTS_TEST --test q.linear --run 1 --top 1 --chr 20 | + | /net/fantasia/home/hmkang/bin/epacts/bin/epacts single --vcf $GCOUT/vcfs/chr20/chr20.filtered.vcf.gz --ped $GCDATA/test.GBR60.ped --out EPACTS_TEST --test q.linear --run 1 --top 1 --chr 20 |
= Tutorial Inputs = | = Tutorial Inputs = | ||
| − | == Alignment Pipeline == | + | == Alignment Pipeline == |
| − | The inputs to the tutorial alignment pipeline are: | + | The command-line inputs to the tutorial alignment pipeline are: |
| − | # [[#Alignment Configuration File|Configuration File (--conf)]] | + | # [[#Alignment Configuration File|Configuration File (--conf)]] |
| − | # [[#Alignment Output Directory|Output Directory (--outdir)]] | + | #* Specifies the configuration file to use when running |
| + | # [[#Alignment Output Directory|Output Directory (--outdir)]] | ||
| + | #* Directory where the output should be placed. | ||
| + | Additional information required to run the alignment pipeline: | ||
| + | # Index file of FASTQs | ||
| + | # Reference files | ||
| + | For the tutorial, these values are specified in the configuration file. | ||
| + | |||
| + | === Alignment Configuration File === | ||
| + | The configuration file contains KEY = VALUE settings that override defaults and set specific values for the given run. | ||
| + | |||
| + | <pre> | ||
| + | INDEX_FILE = GBR60fastq.index | ||
| + | ############ | ||
| + | # References | ||
| + | REF_DIR = chr20Ref | ||
| + | AS = NCBI37 | ||
| + | FA_REF = $(REF_DIR)/human_g1k_v37_chr20.fa | ||
| + | DBSNP_VCF = $(REF_DIR)/dbsnp135_chr20.vcf.gz | ||
| + | HM3_VCF = $(REF_DIR)/hapmap_3.3.b37.sites.chr20.vcf.gz | ||
| + | </pre> | ||
| + | |||
| + | This configuration file sets: | ||
| + | * INDEX_FILE - file containing the fastqs to be processed as well as the read group information for these fastqs. | ||
| − | + | * Reference Information: | |
| − | + | ** AS - assembly value to put in the BAM | |
| + | ** FA_REF - the reference file (.fa extension), the additional files should be at the same location: | ||
| + | *** human_g1k_v37_chr20-bs.umfa | ||
| + | *** human_g1k_v37_chr20.dict | ||
| + | *** human_g1k_v37_chr20.fa | ||
| + | *** human_g1k_v37_chr20.fa.amb | ||
| + | *** human_g1k_v37_chr20.fa.ann | ||
| + | *** human_g1k_v37_chr20.fa.bwt | ||
| + | *** human_g1k_v37_chr20.fa.fai | ||
| + | *** human_g1k_v37_chr20.fa.GCcontent | ||
| + | *** human_g1k_v37_chr20.fa.pac | ||
| + | *** human_g1k_v37_chr20.fa.rbwt | ||
| + | *** human_g1k_v37_chr20.fa.rpac | ||
| + | *** human_g1k_v37_chr20.fa.rsa | ||
| + | *** human_g1k_v37_chr20.fa.sa | ||
| + | ** DBSNP_VCF - a vcf containing the dbsnp positions | ||
| + | ** HM3_VCF - hapmap vcf | ||
| − | + | The index file and chromosome 20 references are included with the example data under the $GCDATA directory. The tutorial uses chromosome 20 only references in order to speed the processing time. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | When you run on your own data, that is more than just chromosome 20, you will need to use the full reference files. Full Reference files can be downloaded from [[GotCloudReference]]. If you are using these reference files, you will only need to specify REF_DIR in your configuration file to the full path to where they are installed. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | When running on your own data, you will also need to update the INDEX_FILE to point to your own index file. See [[#Alignment Index File|Alignment Index File]] for more information on the contents of the index file. | |
| − | + | Note: It is recommended that you use absolute paths (full path names, like “/home/mktrost/gotcloudReference” rather than just “gotcloudReference”). This example does not use absolute paths in order to be flexible to where the data is installed, but using relative paths requires it to be run from the correct directory. | |
| − | This | ||
| − | + | === Alignment Output Directory === | |
| − | + | This setting tells the pipeline where to write the output files. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | The output directory will be created if necessary and will contain the following Directories/files: | ||
| + | * bams - directory containing the final bams/bai files | ||
| + | ** HG00096.OK - indicates that this sample completed alignment processing | ||
| + | ** HG00100.OK - indicates that this sample completed alignment processing | ||
| + | * failLogs - directory containing logs from steps that failed | ||
| + | * Makefiles - directory containing the makefiles with commands for processing each sample | ||
| + | ** biopipe_HG00096.Makefile | ||
| + | ** biopipe_HG00100.Makefile | ||
| + | ** biopipe_HG00096.Makefile.log – log file from running the associated Makefiles | ||
| + | ** biopipe_HG00100.Makefile.log – log file from running the associated Makefiles | ||
| + | * QCFiles - directory containing the QC Results | ||
| + | ** | ||
| + | * tmp - directory containing temporary alignment files | ||
| + | ** | ||
| − | |||
| − | |||
| − | |||
| − | ===Index file=== | + | ===Index file=== |
| − | There are four fastq files in {ROOT_DIR}/test/align/fastq/Sample_1 and four fastq files in {ROOT_DIR}/test/align/fastq/Sample_2, both in paired-end format. Normally, we would need to build an index file for these files. Conveniently, an index file (indexFile.txt) already exists for the automatic test samples. It can be found in {ROOT_DIR}/test/align/, and contains the following information in tab-delimited format: | + | There are four fastq files in {ROOT_DIR}/test/align/fastq/Sample_1 and four fastq files in {ROOT_DIR}/test/align/fastq/Sample_2, both in paired-end format. Normally, we would need to build an index file for these files. Conveniently, an index file (indexFile.txt) already exists for the automatic test samples. It can be found in {ROOT_DIR}/test/align/, and contains the following information in tab-delimited format: |
| − | MERGE_NAME FASTQ1 FASTQ2 RGID SAMPLE LIBRARY CENTER PLATFORM | + | MERGE_NAME FASTQ1 FASTQ2 RGID SAMPLE LIBRARY CENTER PLATFORM |
| − | Sample1 fastq/Sample_1/File1_R1.fastq.gz fastq/Sample_1/File1_R2.fastq.gz RGID1 SampleID1 Lib1 UM ILLUMINA | + | Sample1 fastq/Sample_1/File1_R1.fastq.gz fastq/Sample_1/File1_R2.fastq.gz RGID1 SampleID1 Lib1 UM ILLUMINA |
| − | Sample1 fastq/Sample_1/File2_R1.fastq.gz fastq/Sample_1/File2_R2.fastq.gz RGID1a SampleID1 Lib1 UM ILLUMINA | + | Sample1 fastq/Sample_1/File2_R1.fastq.gz fastq/Sample_1/File2_R2.fastq.gz RGID1a SampleID1 Lib1 UM ILLUMINA |
| − | Sample2 fastq/Sample_2/File1_R1.fastq.gz fastq/Sample_2/File1_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA | + | Sample2 fastq/Sample_2/File1_R1.fastq.gz fastq/Sample_2/File1_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA |
| − | Sample2 fastq/Sample_2/File2_R1.fastq.gz fastq/Sample_2/File2_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA | + | Sample2 fastq/Sample_2/File2_R1.fastq.gz fastq/Sample_2/File2_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA |
If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you prefer, you can create a new index file and change the MERGE_NAME, RGID, SAMPLE, LIBRARY, CENTER, or PLATFORM values. It is recommended that you do not modify existing files in {ROOT_DIR}/test/align. | If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you prefer, you can create a new index file and change the MERGE_NAME, RGID, SAMPLE, LIBRARY, CENTER, or PLATFORM values. It is recommended that you do not modify existing files in {ROOT_DIR}/test/align. | ||
| − | If you want to run this example from a different directory, make sure the FASTQ1 and FASTQ2 paths are correct. That is, each of the FASTQ1 and FASTQ2 entry in the index file should look like the following: | + | If you want to run this example from a different directory, make sure the FASTQ1 and FASTQ2 paths are correct. That is, each of the FASTQ1 and FASTQ2 entry in the index file should look like the following: |
{ROOT_DIR}/test/align/fastq/Sample_1/File1_R1.fastq.gz | {ROOT_DIR}/test/align/fastq/Sample_1/File1_R1.fastq.gz | ||
| − | Alternately, if you want to run this example from a different directory, but do not want to edit the index file, you can create a relative path to the test fastq files so their path agrees with that listed in the index file: | + | Alternately, if you want to run this example from a different directory, but do not want to edit the index file, you can create a relative path to the test fastq files so their path agrees with that listed in the index file: |
| − | ln -s {ROOT_DIR}/test/align/fastq fastq | + | ln -s {ROOT_DIR}/test/align/fastq fastq |
| − | This will create a symbolic link to the test fastq directory from your current directory. | + | This will create a symbolic link to the test fastq directory from your current directory. |
| − | (More information about: [[Mapping_Pipeline#Sequence_Index_File|the index file]].) | + | (More information about: [[Mapping_Pipeline#Sequence_Index_File|the index file]].) |
| − | ===Configuration file=== | + | ===Configuration file=== |
| − | Similar to the index file, a configuration file (test.conf) already exists for the automatic test samples. It contains the following information: | + | Similar to the index file, a configuration file (test.conf) already exists for the automatic test samples. It contains the following information: |
| − | INDEX_FILE = indexFile.txt | + | INDEX_FILE = indexFile.txt |
| − | ############ | + | ############ |
| − | # References | + | # References |
| − | REF_DIR = $(PIPELINE_DIR)/test/align/chr20Ref | + | REF_DIR = $(PIPELINE_DIR)/test/align/chr20Ref |
| − | AS = NCBI37 | + | AS = NCBI37 |
| − | FA_REF = $(REF_DIR)/human_g1k_v37_chr20.fa | + | FA_REF = $(REF_DIR)/human_g1k_v37_chr20.fa |
| − | DBSNP_VCF = $(REF_DIR)/dbsnp.b130.ncbi37.chr20.vcf.gz | + | DBSNP_VCF = $(REF_DIR)/dbsnp.b130.ncbi37.chr20.vcf.gz |
| − | PLINK = $(REF_DIR)/hapmap_3.3.b37.chr20 | + | PLINK = $(REF_DIR)/hapmap_3.3.b37.chr20 |
If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you are using a different index file, make sure your index file is named correctly in the first line. If you are not running this from {ROOT_DIR}/test/align, make sure your configuration and index files are in the same directory. | If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you are using a different index file, make sure your index file is named correctly in the first line. If you are not running this from {ROOT_DIR}/test/align, make sure your configuration and index files are in the same directory. | ||
| − | (More information about: [[Mapping_Pipeline#Reference_Files|reference files]], [[Mapping_Pipeline#Optional_Configurable_Settings|optional configurable settings]], or [[Mapping_Pipeline#Command-Line_Options|command-line options]].) | + | (More information about: [[Mapping_Pipeline#Reference_Files|reference files]], [[Mapping_Pipeline#Optional_Configurable_Settings|optional configurable settings]], or [[Mapping_Pipeline#Command-Line_Options|command-line options]].) |
| − | ===Running the alignment pipeline=== | + | ===Running the alignment pipeline=== |
| − | You are now ready to run the alignment pipeline. | + | You are now ready to run the alignment pipeline. |
| − | To run the alignment pipeline, enter the following command: | + | To run the alignment pipeline, enter the following command: |
| − | {ROOT_DIR}/bin/gen_biopipeline.pl -conf test.conf -out_dir {OUT_DIR} | + | {ROOT_DIR}/bin/gen_biopipeline.pl -conf test.conf -out_dir {OUT_DIR} |
| − | where {OUT_DIR} is the directory in which you wish to store the resulting BAM files (for example, ~/out). | + | where {OUT_DIR} is the directory in which you wish to store the resulting BAM files (for example, ~/out). |
| − | If everything went well, you will see the following messages: | + | If everything went well, you will see the following messages: |
| − | Created {OUT_DIR}/Makefiles/biopipe_Sample2.Makefile | + | Created {OUT_DIR}/Makefiles/biopipe_Sample2.Makefile |
| − | Created {OUT_DIR}/Makefiles/biopipe_Sample1.Makefile | + | Created {OUT_DIR}/Makefiles/biopipe_Sample1.Makefile |
| − | --------------------------------------------------------------------- | + | --------------------------------------------------------------------- |
| − | Submitted 2 commands | + | Submitted 2 commands |
| − | Waiting for commands to complete... . . Commands finished in 33 secs with no errors reported | + | Waiting for commands to complete... . . Commands finished in 33 secs with no errors reported |
| − | The aligned BAM files are found in {OUT_DIR}/alignment.recal/ | + | The aligned BAM files are found in {OUT_DIR}/alignment.recal/ |
| − | ==Analyzing a Sample== | + | ==Analyzing a Sample== |
| − | Using UMAKE, you can analyze BAM files by calling SNPs, and generate a VCF file containing the results. Once again, we can analyze BAM files used in the automatic test. For this example, we have 60 BAM files, which can be found in {ROOT_DIR}/test/umake/bams. These contain sequence information for a targeted region in chromosome 20 | + | Using UMAKE, you can analyze BAM files by calling SNPs, and generate a VCF file containing the results. Once again, we can analyze BAM files used in the automatic test. For this example, we have 60 BAM files, which can be found in {ROOT_DIR}/test/umake/bams. These contain sequence information for a targeted region in chromosome 20. |
| − | |||
| − | |||
| + | In addition to the BAM files, you will need three files to run UMAKE: an index file, a configuration file, and a bed file (needed to analyze BAM files from targeted/exome sequencing). | ||
| + | |||
| − | ===Index file=== | + | ===Index file=== |
| − | First, you need a list of all the BAM files to be analyzed. Conveniently, the a test index file (umake_test.index) already exists in {ROOT_DIR}/test/umake/. It contains the following information: | + | First, you need a list of all the BAM files to be analyzed. Conveniently, the a test index file (umake_test.index) already exists in {ROOT_DIR}/test/umake/. It contains the following information: |
| − | NA12272 ALL bams/NA12272.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam | + | NA12272 ALL bams/NA12272.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam |
| − | NA12004 ALL bams/NA12004.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam | + | NA12004 ALL bams/NA12004.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam |
| − | ... | + | ... |
| − | NA12874 ALL bams/NA12874.mapped.LS454.ssaha2.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam | + | NA12874 ALL bams/NA12874.mapped.LS454.ssaha2.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam |
| − | You can use this file directly if you change your current directory to {ROOT_DIR}/test/umake/. | + | You can use this file directly if you change your current directory to {ROOT_DIR}/test/umake/. |
| − | Alternately, if you want to copy and use this index file to a different directory, you can create a symbolic link to the bams folder as follows: | + | Alternately, if you want to copy and use this index file to a different directory, you can create a symbolic link to the bams folder as follows: |
| − | ln -s {ROOT_DIR}/test/umake/bams bams | + | ln -s {ROOT_DIR}/test/umake/bams bams |
| − | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Index_File|the index file]].) | + | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Index_File|the index file]].) |
| − | ===BED file=== | + | ===BED file=== |
| − | This file contains a single line: | + | This file contains a single line: |
| − | chr20 20000050 20300000 | + | chr20 20000050 20300000 |
| − | You can copy this to the current directory and use it as-is. | + | You can copy this to the current directory and use it as-is. |
| − | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Targeted.2FExome_Sequencing_Settings|targeted/exome sequencing settings]].) | + | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Targeted.2FExome_Sequencing_Settings|targeted/exome sequencing settings]].) |
| − | ===Configuration file=== | + | ===Configuration file=== |
| − | A configuration file (umake_test.conf) already exists in {ROOT_DIR}/test/umake/. It contains the following information: | + | A configuration file (umake_test.conf) already exists in {ROOT_DIR}/test/umake/. It contains the following information: |
| − | CHRS = 20 | + | CHRS = 20 |
| − | TEST_ROOT = $(UMAKE_ROOT)/test/umake | + | TEST_ROOT = $(UMAKE_ROOT)/test/umake |
| − | BAM_INDEX = $(TEST_ROOT)/umake_test.index | + | BAM_INDEX = $(TEST_ROOT)/umake_test.index |
| − | OUT_PREFIX = umake_test | + | OUT_PREFIX = umake_test |
| − | REF_ROOT = $(TEST_ROOT)/ref | + | REF_ROOT = $(TEST_ROOT)/ref |
| − | # | + | # |
| − | REF = $(REF_ROOT)/karma.ref/human.g1k.v37.chr20.fa | + | REF = $(REF_ROOT)/karma.ref/human.g1k.v37.chr20.fa |
| − | INDEL_PREFIX = $(REF_ROOT)/indels/1kg.pilot_release.merged.indels.sites.hg19 | + | INDEL_PREFIX = $(REF_ROOT)/indels/1kg.pilot_release.merged.indels.sites.hg19 |
| − | DBSNP_PREFIX = $(REF_ROOT)/dbSNP/dbsnp_135_b37.rod | + | DBSNP_PREFIX = $(REF_ROOT)/dbSNP/dbsnp_135_b37.rod |
| − | HM3_PREFIX = $(REF_ROOT)/HapMap3/hapmap3_r3_b37_fwd.consensus.qc.poly | + | HM3_PREFIX = $(REF_ROOT)/HapMap3/hapmap3_r3_b37_fwd.consensus.qc.poly |
| − | # | + | # |
| − | RUN_INDEX = TRUE # create BAM index file | + | RUN_INDEX = TRUE # create BAM index file |
| − | RUN_PILEUP = TRUE # create GLF file from BAM | + | RUN_PILEUP = TRUE # create GLF file from BAM |
| − | RUN_GLFMULTIPLES = TRUE # create unfiltered SNP calls | + | RUN_GLFMULTIPLES = TRUE # create unfiltered SNP calls |
| − | RUN_VCFPILEUP = TRUE # create PVCF files using vcfPileup and run infoCollector | + | RUN_VCFPILEUP = TRUE # create PVCF files using vcfPileup and run infoCollector |
| − | RUN_FILTER = TRUE # filter SNPs using vcfCooker | + | RUN_FILTER = TRUE # filter SNPs using vcfCooker |
| − | RUN_SPLIT = TRUE # split SNPs into chunks for genotype refinement | + | RUN_SPLIT = TRUE # split SNPs into chunks for genotype refinement |
| − | RUN_BEAGLE = FALSE # BEAGLE - MUST SET AFTER FINISHING PREVIOUS STEPS | + | RUN_BEAGLE = FALSE # BEAGLE - MUST SET AFTER FINISHING PREVIOUS STEPS |
| − | RUN_SUBSET = FALSE # SUBSET FOR THUNDER - MAY BE SET WITH BEAGLE STEP TOGETHER | + | RUN_SUBSET = FALSE # SUBSET FOR THUNDER - MAY BE SET WITH BEAGLE STEP TOGETHER |
| − | RUN_THUNDER = FALSE # THUNDER - MUST SET AFTER FINISHING PREVIOUS STEPS | + | RUN_THUNDER = FALSE # THUNDER - MUST SET AFTER FINISHING PREVIOUS STEPS |
| − | ############################################################################### | + | ############################################################################### |
| − | WRITE_TARGET_LOCI = TRUE # FOR TARGETED SEQUENCING ONLY -- Write loci file when performing pileup | + | WRITE_TARGET_LOCI = TRUE # FOR TARGETED SEQUENCING ONLY -- Write loci file when performing pileup |
| − | UNIFORM_TARGET_BED = $(TEST_ROOT)/umake_test.bed # Targeted sequencing : When all individuals has the same target. Otherwise, comment it out | + | UNIFORM_TARGET_BED = $(TEST_ROOT)/umake_test.bed # Targeted sequencing : When all individuals has the same target. Otherwise, comment it out |
| − | OFFSET_OFF_TARGET = 50 # Extend target by given # of bases | + | OFFSET_OFF_TARGET = 50 # Extend target by given # of bases |
| − | MULTIPLE_TARGET_MAP = # Target per individual : Each line contains [SM_ID] [TARGET_BED] | + | MULTIPLE_TARGET_MAP = # Target per individual : Each line contains [SM_ID] [TARGET_BED] |
| − | TARGET_DIR = target # Directory to store target information | + | TARGET_DIR = target # Directory to store target information |
| − | SAMTOOLS_VIEW_TARGET_ONLY = TRUE # When performing samtools view, exclude off-target regions (may make command line too long) | + | SAMTOOLS_VIEW_TARGET_ONLY = TRUE # When performing samtools view, exclude off-target regions (may make command line too long) |
| − | If you are running this from a different directory, you will want to change some of the lines as follows: | + | If you are running this from a different directory, you will want to change some of the lines as follows: |
| − | BAM_INDEX = {CURRENT_DIR}/umake_test.index | + | BAM_INDEX = {CURRENT_DIR}/umake_test.index |
| − | UNIFORM_TARGET_BED = {CURRENT_DIR}/umake_test.bed | + | UNIFORM_TARGET_BED = {CURRENT_DIR}/umake_test.bed |
where {CURRENT_DIR} is the absolute path to the directory that contains the index and bed files. | where {CURRENT_DIR} is the absolute path to the directory that contains the index and bed files. | ||
| − | An additional option can be added in the configuration file: | + | An additional option can be added in the configuration file: |
| − | OUT_DIR = {OUT_DIR} | + | OUT_DIR = {OUT_DIR} |
| − | where {OUT_DIR} is the name of directory in which you want the output to be stored. If you do not specify this in the configuration file, you will need to add an extra parameter when you run UMAKE in the next step. | + | where {OUT_DIR} is the name of directory in which you want the output to be stored. If you do not specify this in the configuration file, you will need to add an extra parameter when you run UMAKE in the next step. |
| − | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Configuration_File|the configuration file]], [[Variant_Calling_Pipeline_(UMAKE)#Reference_Files|reference files]].) | + | (More information about: [[Variant_Calling_Pipeline_(UMAKE)#Configuration_File|the configuration file]], [[Variant_Calling_Pipeline_(UMAKE)#Reference_Files|reference files]].) |
| − | ===Running UMAKE=== | + | ===Running UMAKE=== |
| − | If you added an OUT_DIR line to the configuration file, you can run UMAKE with the following command: | + | If you added an OUT_DIR line to the configuration file, you can run UMAKE with the following command: |
| − | {ROOT_DIR}/bin/umake.pl --conf umake_test.conf --snpcall --numjobs 2 | + | {ROOT_DIR}/bin/umake.pl --conf umake_test.conf --snpcall --numjobs 2 |
| − | If you have not added an OUT_DIR line to the configuration file, you can specify the output directory directly with the following command: | + | If you have not added an OUT_DIR line to the configuration file, you can specify the output directory directly with the following command: |
| − | {ROOT_DIR}/bin/umake.pl --conf umake_test.conf --outdir {OUT_DIR} --snpcall --numjobs 2 | + | {ROOT_DIR}/bin/umake.pl --conf umake_test.conf --outdir {OUT_DIR} --snpcall --numjobs 2 |
| − | where {OUT_DIR} is the directory in which you want the output to be stored. | + | where {OUT_DIR} is the directory in which you want the output to be stored. |
| − | Either command will perform SNP calling on the test samples. If you find the resulting VCF files located in {OUT_DIR}/vcfs/chr20, then you have successfully called the SNPs from the test BAM files. | + | Either command will perform SNP calling on the test samples. If you find the resulting VCF files located in {OUT_DIR}/vcfs/chr20, then you have successfully called the SNPs from the test BAM files. |
| − | ==Further Information== | + | ==Further Information== |
[[Mapping_Pipeline|Mapping (Alignment) Pipeline]] | [[Mapping_Pipeline|Mapping (Alignment) Pipeline]] | ||
[[Variant_Calling_Pipeline_(UMAKE)|Variant Calling Pipeline (UMAKE)]] | [[Variant_Calling_Pipeline_(UMAKE)|Variant Calling Pipeline (UMAKE)]] | ||
Revision as of 15:49, 4 March 2013
GotCloud Tutorial
In this tutorial, we illustrate some of the essential steps in the analysis of next generation sequence data.
Analysis starts with FASTQ files, the typical format provided from your sequencing center containing the sequence & base quality information for your data.
The fastq files are processed using the alignment pipeline which finds the most likely genomic location for each read and stores that information in a BAM (Binary Sequence Alignment/Map format) file. In addition to the sequence and base quality information contained in FASTQ files, a BAM file also contains the genomic location and some additional information about the mapping. As part of the alignment pipeline, the base qualities are adjusted to more accurately reflect the likelihood that the base is correct.
The variant calling pipeline processes the BAMs file produced by the alignment pipeline, generating an initial list of polymorphic sites and genotypes stored in a VCF (Variant Call Format) file and then uses haplotype information to refine these genotypes in an updated VCF file.
After variant calling, there is an optional step to further filter the variants using a Support Vector Machine (SVM). This feature is in development and will soon be added to gotcloud and this tutorial.
This tutorial then demonstrates how EPACTS (Efficient and Parallelizable Association Container Toolbox) can be used to perform statistical tests to identify genome-wide association from sequence data.
GotCloud incorporates the alignment and variant calling pipelines into one easy to use tool. GotCloud can run on a user's computer, on an instance in a compute cloud, and can even split the work up onto a cluster of machines or instances. This tutorial is just a small test that just runs on the machine the commands are run on.
STEP 1 : Setup GotCloud
GotCloud has been developed and tested on Linux Ubuntu 12.10 and 12.04.2 LTS but has not been tested on other Linux operating systems. It is not available for Windows. If you do not have your own set of machines to run on, GotCloud is also available for Ubuntu running on the Amazon Elastic Compute Cloud, see Amazon_Snapshot for more information.
Step 1a: Setup Environment
We will use 3 different directories for this tutorial and will use the following variables to stand for these directories:
- $GCHOME : path to the directory where gotcloud is installed
- $GCDATA : path to the directory where the example data is installed
- $GCOUT : path to your output directory
The following steps will set these environment variables in your Linux terminal allowing you to type $GCHOME to specify the path to gotcloud instead of having to type the entire path (absolute path).
Note: These settings will be local to that specific terminal. If you open a new terminal you will need to set the variables again in that terminal.
There are two different ways to set these variables and they depend on what type of shell your terminal is using. The shell you are using does not matter, other than for using the appropriate commands for setting the variables. To determine which shell your terminal is running, the following command will display your shell type, so type the following in your terminal:
ps -p $$ -ocomm=
If your shell is bash or sh, set your variables using:
export GCHOME=~/gotcloud export GCDATA=~/gotcloudExample export GCOUT=~/gotcloudTutorial
If your shell is csh, tcsh, set your variables using:
setenv GCHOME ~/gotcloud setenv GCDATA ~/gotcloudExample setenv GCOUT ~/gotcloudTutorial
If the directories specified above do not reflect where gotcloud and/or the example data is installed or where you want your output to go, then replace those directories with the full paths to the appropriate directories.
After setting these variables, you can copy and paste the rest of the commands in the tutorial. If you do not want to use variables, you can type the commands in with the appropriate paths specified.
Step 1b: Install GotCloud
In order to run this tutorial, you need to make sure you have GotCloud installed on your system.
If you have root and would like to install gotcloud on your system, follow: root access installation instructions
Otherwise, you can install it in your own directory:
- Create & change to the directory where you want gotcloud installed
- Download the gotcloud tar from the ftp site.
- Extract the tar
- Build (compile) the source
- Note: as the source builds, many messages will scroll through your terminal. You may even see some warnings. These messages are normal and expected. As long as the build does not end with an error, you have successfully built the source.
mkdir -p $GCHOME; cd $GCHOME wget ftp://share.sph.umich.edu/gotcloud/gotcloud.tar # Download tar xvf gotcloud.tar --strip 1 # Extract cd $GCHOME/src; make # Build source
GotCloud requires the following tools to be installed. You can run $GCHOME/scripts/check_requirements.sh ...TBD – put in required programs/tools.
- java (java-common default-jre on ubuntu)
- make (make on ubuntu)
- libssl (libssl0.9.8 on ubuntu)
Step 1c: Install Example Dataset
Our dataset consists of 60 individuals from Great Britain (GBR) sequenced by the 1000 Genomes Project. These individuals have been sequenced to an average depth of about 4x.
To conserve time and disk-space, our analysis will focus on a small region on chromosome 20, 42900000 - 43200000.
The tutorial will run the alignment pipeline on 2 of the individuals (HG00096, HG00100). The fastqs used for this step are reduced to reads that fall into our target region.
The tutorial will then used previously aligned/mapped reads for the full 60 individuals to generate a list of polymorphic sites and estimate accurate genotypes at each of these sites.
The example dataset we'll be using is available at: ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar
- Create & Change directory to where you want to install the Tutorail data
- Download the dataset tar from the ftp site
- Extract the tar
mkdir -p $GCDATA; cd $GCDATA wget ftp://share.sph.umich.edu/gotcloud/gotcloudExample.tar # Download tar xvf gotcloudExample.tar --strip 1 # Extract
STEP 2 : Run GotCloud Alignment Pipeline
The first step in processing next generation sequence data is mapping the reads to the reference genome, generating per sample BAM files.
The alignment pipeline has multiple built-in steps to generate BAMs:
- Align the fastqs to the reference genome
- handles both single & paired end
- Merge the results from multiple fastqs into 1 file per sample
- Mark Duplicate Reads are marked
- Recalibrate Base Qualities
This processing results in 1 BAM file per sample.
The alignment pipeline also includes Quality Control (QC) steps:
- Visualization of various quality measures (QPLOT)
- Screen for sample contamination & swap (VerifyBamID)
Run the alignment pipeline (the example aligns 2 samples) :
$GCHOME/gotcloud align --conf $GCDATA/GBR60align.conf --outdir $GCOUT
Upon successful completion of the alignment pipeline (about 1-2 minutes), you will see the following message:
Processing finished in nn secs with no errors reported
The final BAM files produced by the alignment pipeline are:
ls $GCOUT/bams
In this directory you will see:
- BAM (.bam) files - 1 per sample
- HG00096.recal.bam
- HG00100.recal.bam
- BAM index files (.bai) – 1 per sample
- HG00096.recal.bam.bai
- HG00100.recal.bam.bai
- BAM checksum files (.md5) – 1 per sample
- HG00096.recal.bam.md5
- HG00100.recal.bam.md5
- Indicator flies that the step completed successfully:
- HG00096.recal.bam.done
- HG00100.recal.bam.done
The Quality Control (QC) files are:
ls $GCOUT/QCFiles
In this directory you will see:
- VerifyBamID output files:
- HG00096.genoCheck.depthRG
- HG00096.genoCheck.depthSM
- HG00096.genoCheck.selfRG
- HG00096.genoCheck.selfSM
- HG00100.genoCheck.depthRG
- HG00100.genoCheck.depthSM
- HG00100.genoCheck.selfRG
- HG00100.genoCheck.selfSM
- VerifyBamID step completion files – 1 per sample
- HG00096.genoCheck.done
- HG00100.genoCheck.done
- QPLOT output files
- HG00096.qplot.R
- HG00096.qplot.stats
- HG00100.qplot.R
- HG00100.qplot.stats
- QPLOT step completion files – 1 per sample
- HG00096.qplot.done
- HG00100.qplot.done
For information on the VerifyBamID output, see: Understanding VerifyBamID output
For information on the QPLOT output, see: Understanding QPLOT output
STEP 3 : Run GotCloud Variant Calling Pipeline
The next step is to analyze BAM files by calling SNPs and generating a VCF file containing the variant calls.
The variant calling pipeline has multiple built-in steps to generate BAMs:
- Filter out reads with low mapping quality
- Per Base Alignment Quality Adjustment (BAQ)
- Resolve overlapping paired end reads
- Generate genotype likelihood files
- Perform variant calling
- Extract features from variant sites
- Perform variant filtering
To speed variant calling, each chromosome is broken up into smaller regions which are processed separately. While initially split by sample, the per sample data gets merged and is processed together for each region. These regions are later merged to result in a single Variant Call File (VCF) per chromosome.
Run the variant calling pipeline:
$GCHOME/gotcloud snpcall --conf GBR60vc.conf --outdir $GCOUT --numjobs 2 --region 20:42900000-43200000
Upon successful completion of the variant calling pipeline (about 3-4 minutes), you will see the following message:
Commands finished in nnn secs with no errors reported
On SNP Call success, the VCF files of interest are:
ls $GCOUT/vcfs/chr20/chr20.filtered*
This gives you the following files:
- chr20.filtered.vcf.gz - vcf for whole chromosome after it has been run through filters and marked with PASS/FAIL including per sample information
- chr20.filtered.sites.vcf - vcf for whole chromosome after it has been run through filters and marked with PASS/FAIL without the per sample information
- chr20.filtered.sites.vcf.log - log file
- chr20.filtered.sites.vcf.summary - summary of filters applied
- chr20.filtered.vcf.gz.OK - indicator that the filtering completed successfully
- chr20.filtered.vcf.gz.tbi - index file for the vcf file
Also in the $GCOUT/vcfs/chr20 directory are intermediate files:
- the whole chromosome variant calls prior to any filtering:
- chr20.merged.sites.vcf - no sample information
- chr20.merged.stats.vcf
- chr20.merged.vcf - includes sample information
- chr20.merged.vcf.OK - indicator that the step completed successfully
- 40000001.45000000 subdirectory contains the data for just that region.
The $GCOUT/split/chr20 folder contains a VCF with just the sites that pass the filters.
Ls $GCOUT/split/chr20/
- chr20.filtered.PASS.vcf.gz – vcf of just sites that pass all filters
- chr20.filtered.PASS.split.1.vcf.gz - intermediate file
- chr20.filtered.PASS.split.err - log file
- chr20.filtered.PASS.split.vcflist - list of intermediate files
- subset.OK
In addition to the vcfs subdirectory, there are additional intermediate files/directories:
- glfs – holds genotype likelihood format GLF files split by chromosome, region, and sample
- pvcfs – holds intermediate vcf files split by chromosome and region
Note: the tutorial does not produce a target directory, but if you run with targeted data, you may see that.
STEP 4 : Run GotCloud Genotype Refinement Pipeline
The next step is to perform genotype refinement using linkage disequilibrium information using Beagle & ThunderVCF.
Run the LD-aware genotype refinement pipeline:
$GCHOME/gotcloud ldrefine --conf GBR60vc.conf --outdir $GCOUT --numjobs 2
Upon successful completion of this pipeline, you will see the following message:
Commands finished in nnn secs with no errors reported
The output from the beagle step of the genotype refinement pipeline is found in:
ls $GCOUT/beagle/chr20/chr20.filtered.PASS.beagled.vcf.gz $GCOUT/beagle/chr20/chr20.filtered.PASS.beagled.vcf.gz.tbi
The output from the thunderVcf (final step) of the genotype refinement pipeline is found in:
ls $GCOUT/thunder/chr20/GBR/chr20.filtered.PASS.beagled.GBR.thunder.vcf.gz $GCOUT/thunder/chr20/GBR/chr20.filtered.PASS.beagled.GBR.thunder.vcf.gz.tbi
STEP 5 : Run Support Vector Machine (SVM) Pipeline
STEP 6 : Run GotCloud Association Analysis Pipeline
/net/fantasia/home/hmkang/bin/epacts/bin/epacts single --vcf $GCOUT/vcfs/chr20/chr20.filtered.vcf.gz --ped $GCDATA/test.GBR60.ped --out EPACTS_TEST --test q.linear --run 1 --top 1 --chr 20
Tutorial Inputs
Alignment Pipeline
The command-line inputs to the tutorial alignment pipeline are:
- Configuration File (--conf)
- Specifies the configuration file to use when running
- Output Directory (--outdir)
- Directory where the output should be placed.
Additional information required to run the alignment pipeline:
- Index file of FASTQs
- Reference files
For the tutorial, these values are specified in the configuration file.
Alignment Configuration File
The configuration file contains KEY = VALUE settings that override defaults and set specific values for the given run.
INDEX_FILE = GBR60fastq.index ############ # References REF_DIR = chr20Ref AS = NCBI37 FA_REF = $(REF_DIR)/human_g1k_v37_chr20.fa DBSNP_VCF = $(REF_DIR)/dbsnp135_chr20.vcf.gz HM3_VCF = $(REF_DIR)/hapmap_3.3.b37.sites.chr20.vcf.gz
This configuration file sets:
- INDEX_FILE - file containing the fastqs to be processed as well as the read group information for these fastqs.
- Reference Information:
- AS - assembly value to put in the BAM
- FA_REF - the reference file (.fa extension), the additional files should be at the same location:
- human_g1k_v37_chr20-bs.umfa
- human_g1k_v37_chr20.dict
- human_g1k_v37_chr20.fa
- human_g1k_v37_chr20.fa.amb
- human_g1k_v37_chr20.fa.ann
- human_g1k_v37_chr20.fa.bwt
- human_g1k_v37_chr20.fa.fai
- human_g1k_v37_chr20.fa.GCcontent
- human_g1k_v37_chr20.fa.pac
- human_g1k_v37_chr20.fa.rbwt
- human_g1k_v37_chr20.fa.rpac
- human_g1k_v37_chr20.fa.rsa
- human_g1k_v37_chr20.fa.sa
- DBSNP_VCF - a vcf containing the dbsnp positions
- HM3_VCF - hapmap vcf
The index file and chromosome 20 references are included with the example data under the $GCDATA directory. The tutorial uses chromosome 20 only references in order to speed the processing time.
When you run on your own data, that is more than just chromosome 20, you will need to use the full reference files. Full Reference files can be downloaded from GotCloudReference. If you are using these reference files, you will only need to specify REF_DIR in your configuration file to the full path to where they are installed.
When running on your own data, you will also need to update the INDEX_FILE to point to your own index file. See Alignment Index File for more information on the contents of the index file.
Note: It is recommended that you use absolute paths (full path names, like “/home/mktrost/gotcloudReference” rather than just “gotcloudReference”). This example does not use absolute paths in order to be flexible to where the data is installed, but using relative paths requires it to be run from the correct directory.
Alignment Output Directory
This setting tells the pipeline where to write the output files.
The output directory will be created if necessary and will contain the following Directories/files:
- bams - directory containing the final bams/bai files
- HG00096.OK - indicates that this sample completed alignment processing
- HG00100.OK - indicates that this sample completed alignment processing
- failLogs - directory containing logs from steps that failed
- Makefiles - directory containing the makefiles with commands for processing each sample
- biopipe_HG00096.Makefile
- biopipe_HG00100.Makefile
- biopipe_HG00096.Makefile.log – log file from running the associated Makefiles
- biopipe_HG00100.Makefile.log – log file from running the associated Makefiles
- QCFiles - directory containing the QC Results
- tmp - directory containing temporary alignment files
Index file
There are four fastq files in {ROOT_DIR}/test/align/fastq/Sample_1 and four fastq files in {ROOT_DIR}/test/align/fastq/Sample_2, both in paired-end format. Normally, we would need to build an index file for these files. Conveniently, an index file (indexFile.txt) already exists for the automatic test samples. It can be found in {ROOT_DIR}/test/align/, and contains the following information in tab-delimited format:
MERGE_NAME FASTQ1 FASTQ2 RGID SAMPLE LIBRARY CENTER PLATFORM Sample1 fastq/Sample_1/File1_R1.fastq.gz fastq/Sample_1/File1_R2.fastq.gz RGID1 SampleID1 Lib1 UM ILLUMINA Sample1 fastq/Sample_1/File2_R1.fastq.gz fastq/Sample_1/File2_R2.fastq.gz RGID1a SampleID1 Lib1 UM ILLUMINA Sample2 fastq/Sample_2/File1_R1.fastq.gz fastq/Sample_2/File1_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA Sample2 fastq/Sample_2/File2_R1.fastq.gz fastq/Sample_2/File2_R2.fastq.gz RGID2 SampleID2 Lib2 UM ILLUMINA
If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you prefer, you can create a new index file and change the MERGE_NAME, RGID, SAMPLE, LIBRARY, CENTER, or PLATFORM values. It is recommended that you do not modify existing files in {ROOT_DIR}/test/align.
If you want to run this example from a different directory, make sure the FASTQ1 and FASTQ2 paths are correct. That is, each of the FASTQ1 and FASTQ2 entry in the index file should look like the following:
{ROOT_DIR}/test/align/fastq/Sample_1/File1_R1.fastq.gz
Alternately, if you want to run this example from a different directory, but do not want to edit the index file, you can create a relative path to the test fastq files so their path agrees with that listed in the index file:
ln -s {ROOT_DIR}/test/align/fastq fastq
This will create a symbolic link to the test fastq directory from your current directory.
(More information about: the index file.)
Configuration file
Similar to the index file, a configuration file (test.conf) already exists for the automatic test samples. It contains the following information:
INDEX_FILE = indexFile.txt ############ # References REF_DIR = $(PIPELINE_DIR)/test/align/chr20Ref AS = NCBI37 FA_REF = $(REF_DIR)/human_g1k_v37_chr20.fa DBSNP_VCF = $(REF_DIR)/dbsnp.b130.ncbi37.chr20.vcf.gz PLINK = $(REF_DIR)/hapmap_3.3.b37.chr20
If you are in the {ROOT_DIR}/test/align directory, you can use this file as-is. If you are using a different index file, make sure your index file is named correctly in the first line. If you are not running this from {ROOT_DIR}/test/align, make sure your configuration and index files are in the same directory.
(More information about: reference files, optional configurable settings, or command-line options.)
Running the alignment pipeline
You are now ready to run the alignment pipeline.
To run the alignment pipeline, enter the following command:
{ROOT_DIR}/bin/gen_biopipeline.pl -conf test.conf -out_dir {OUT_DIR}
where {OUT_DIR} is the directory in which you wish to store the resulting BAM files (for example, ~/out).
If everything went well, you will see the following messages:
Created {OUT_DIR}/Makefiles/biopipe_Sample2.Makefile
Created {OUT_DIR}/Makefiles/biopipe_Sample1.Makefile
---------------------------------------------------------------------
Submitted 2 commands
Waiting for commands to complete... . . Commands finished in 33 secs with no errors reported
The aligned BAM files are found in {OUT_DIR}/alignment.recal/
Analyzing a Sample
Using UMAKE, you can analyze BAM files by calling SNPs, and generate a VCF file containing the results. Once again, we can analyze BAM files used in the automatic test. For this example, we have 60 BAM files, which can be found in {ROOT_DIR}/test/umake/bams. These contain sequence information for a targeted region in chromosome 20.
In addition to the BAM files, you will need three files to run UMAKE: an index file, a configuration file, and a bed file (needed to analyze BAM files from targeted/exome sequencing).
Index file
First, you need a list of all the BAM files to be analyzed. Conveniently, the a test index file (umake_test.index) already exists in {ROOT_DIR}/test/umake/. It contains the following information:
NA12272 ALL bams/NA12272.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam NA12004 ALL bams/NA12004.mapped.ILLUMINA.bwa.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam ... NA12874 ALL bams/NA12874.mapped.LS454.ssaha2.CEU.low_coverage.20101123.chrom20.20000001.20300000.bam
You can use this file directly if you change your current directory to {ROOT_DIR}/test/umake/.
Alternately, if you want to copy and use this index file to a different directory, you can create a symbolic link to the bams folder as follows:
ln -s {ROOT_DIR}/test/umake/bams bams
(More information about: the index file.)
BED file
This file contains a single line:
chr20 20000050 20300000
You can copy this to the current directory and use it as-is.
(More information about: targeted/exome sequencing settings.)
Configuration file
A configuration file (umake_test.conf) already exists in {ROOT_DIR}/test/umake/. It contains the following information:
CHRS = 20 TEST_ROOT = $(UMAKE_ROOT)/test/umake BAM_INDEX = $(TEST_ROOT)/umake_test.index OUT_PREFIX = umake_test REF_ROOT = $(TEST_ROOT)/ref # REF = $(REF_ROOT)/karma.ref/human.g1k.v37.chr20.fa INDEL_PREFIX = $(REF_ROOT)/indels/1kg.pilot_release.merged.indels.sites.hg19 DBSNP_PREFIX = $(REF_ROOT)/dbSNP/dbsnp_135_b37.rod HM3_PREFIX = $(REF_ROOT)/HapMap3/hapmap3_r3_b37_fwd.consensus.qc.poly # RUN_INDEX = TRUE # create BAM index file RUN_PILEUP = TRUE # create GLF file from BAM RUN_GLFMULTIPLES = TRUE # create unfiltered SNP calls RUN_VCFPILEUP = TRUE # create PVCF files using vcfPileup and run infoCollector RUN_FILTER = TRUE # filter SNPs using vcfCooker RUN_SPLIT = TRUE # split SNPs into chunks for genotype refinement RUN_BEAGLE = FALSE # BEAGLE - MUST SET AFTER FINISHING PREVIOUS STEPS RUN_SUBSET = FALSE # SUBSET FOR THUNDER - MAY BE SET WITH BEAGLE STEP TOGETHER RUN_THUNDER = FALSE # THUNDER - MUST SET AFTER FINISHING PREVIOUS STEPS ############################################################################### WRITE_TARGET_LOCI = TRUE # FOR TARGETED SEQUENCING ONLY -- Write loci file when performing pileup UNIFORM_TARGET_BED = $(TEST_ROOT)/umake_test.bed # Targeted sequencing : When all individuals has the same target. Otherwise, comment it out OFFSET_OFF_TARGET = 50 # Extend target by given # of bases MULTIPLE_TARGET_MAP = # Target per individual : Each line contains [SM_ID] [TARGET_BED] TARGET_DIR = target # Directory to store target information SAMTOOLS_VIEW_TARGET_ONLY = TRUE # When performing samtools view, exclude off-target regions (may make command line too long)
If you are running this from a different directory, you will want to change some of the lines as follows:
BAM_INDEX = {CURRENT_DIR}/umake_test.index
UNIFORM_TARGET_BED = {CURRENT_DIR}/umake_test.bed
where {CURRENT_DIR} is the absolute path to the directory that contains the index and bed files.
An additional option can be added in the configuration file:
OUT_DIR = {OUT_DIR}
where {OUT_DIR} is the name of directory in which you want the output to be stored. If you do not specify this in the configuration file, you will need to add an extra parameter when you run UMAKE in the next step.
(More information about: the configuration file, reference files.)
Running UMAKE
If you added an OUT_DIR line to the configuration file, you can run UMAKE with the following command:
{ROOT_DIR}/bin/umake.pl --conf umake_test.conf --snpcall --numjobs 2
If you have not added an OUT_DIR line to the configuration file, you can specify the output directory directly with the following command:
{ROOT_DIR}/bin/umake.pl --conf umake_test.conf --outdir {OUT_DIR} --snpcall --numjobs 2
where {OUT_DIR} is the directory in which you want the output to be stored.
Either command will perform SNP calling on the test samples. If you find the resulting VCF files located in {OUT_DIR}/vcfs/chr20, then you have successfully called the SNPs from the test BAM files.